Latest News
Why XRF is Critical for Lithium Miners (Even if it Doesn’t Measure the Li)
Lithium mining has shifted from discovery to optimization. As operators process lower-grade, more complex deposits, operating margins and processing efficiency depend on precise chemical control across the flowsheet. At first glance, X-ray fluorescence (XRF) may appear ill-suited because it cannot directly measure lithium in fused samples. Yet recovery is rarely limited by lithium concentration alone. It is governed by the surrounding element matrix, including iron, magnesium, aluminium, silicon, sulfur, and trace elements that influence roasting, leaching, and impurity control. XRF measures the chemistry that ultimately controls reaction stability, impurity behavior, and yield. Even without directly measuring lithium, it provides the elemental visibility required to protect recovery an
...
Optimizing Rare Earth Element (REE) Recovery with XRF Sample Fusion Prep
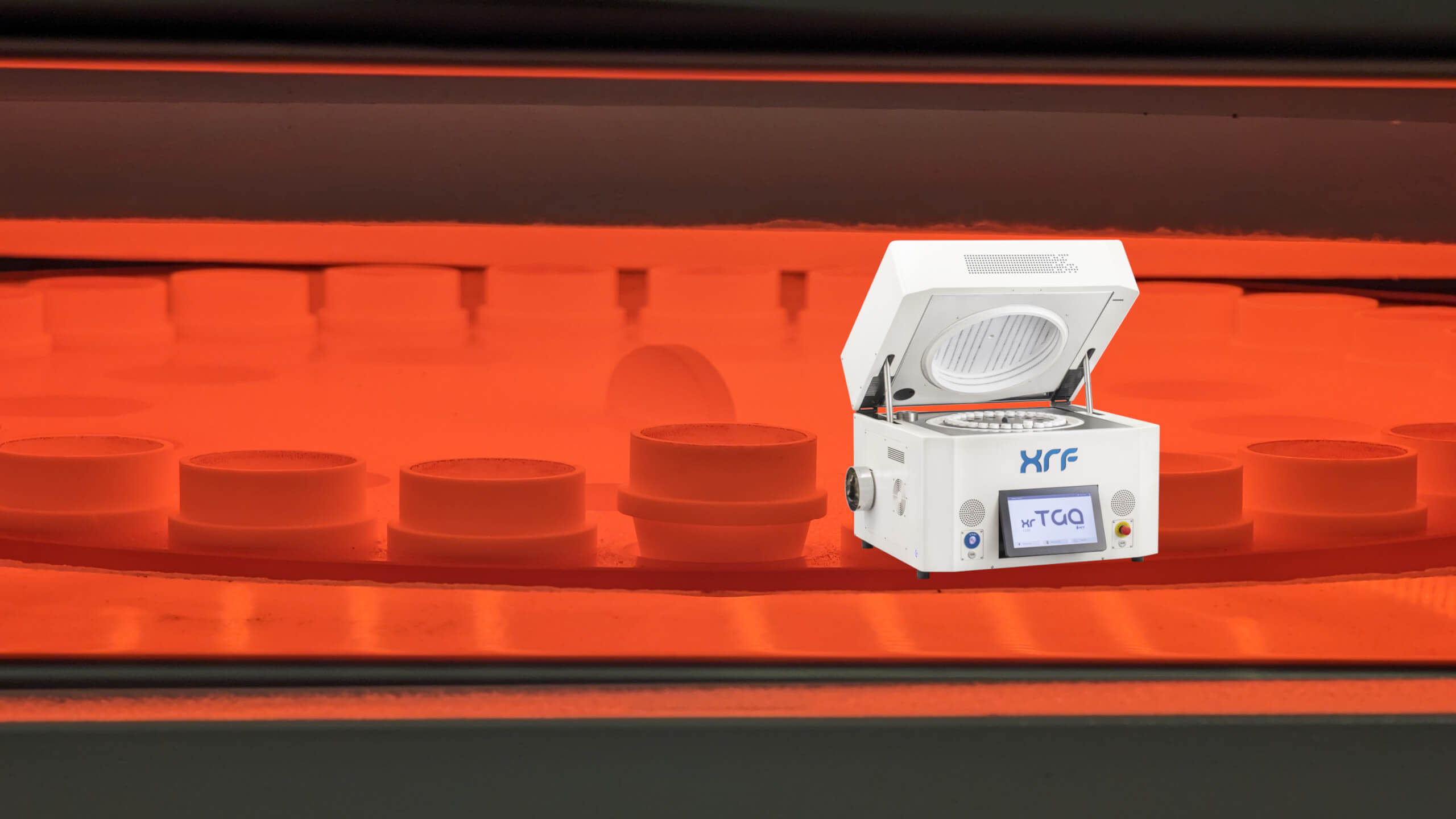
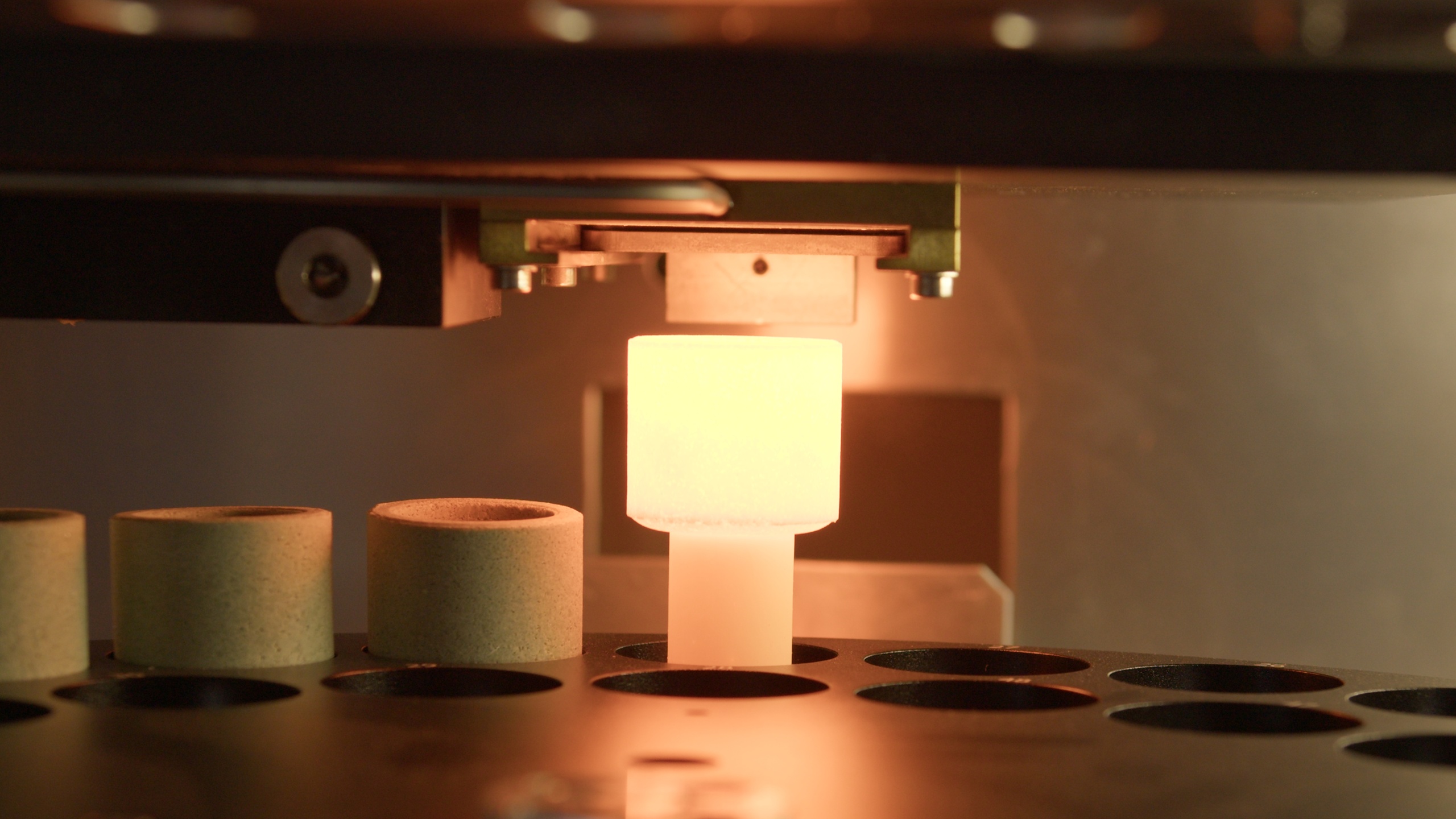
Rare Earth Elements (REEs) are seldom distributed evenly within an ore body. They occur in discrete, often refractory mineral phases where crystal structure and particle size directly influence analytical response. Conventional pressed powder pellets prepared from finely ground ore samples can struggle to represent this heterogeneity, introducing mineralogical and particle-size bias into X-ray fluorescence (XRF) data used for grade control and metallurgical planning. Across the rare earth value chain, incremental recovery gains depend on removing this analytical uncertainty. Fusion-based XRF sample preparation dissolves the mineral lattice into a homogeneous glass bead, delivering precision and comparability that enable tighter process control, improved recovery forecasting, and more ac
...
Tantalum Internal Standards for Copper Analysis – a New Industry Standard?
Global copper demand is rising, driven by electrification, grid storage, and renewable infrastructure. However, the copper being processed is increasingly variable, reflecting deeper deposits, blended feeds, and more complex mineral chemistry. Copper analysis now carries strategic weight within mining operations because laboratory data defines recovery targets, concentrate specifications, and financial reconciliation models. As feed composition shifts, analytical variability can propagate directly into production forecasts and revenue calculations, particularly when matrix effects modify the measured copper fluorescence intensity in X-ray fluorescence (XRF) analysis. To address distortion of copper signal intensity caused by absorption and secondary enhancement effects associated
...