Platinum Products for Electrochemical Analysis
Electrochemical analysis is a complex field of study concerning chemical reactions under electrical stimulation. This involves the transfer of electrons through the heterogeneous interface between an electrode and an ionic conductor. By analyzing the gain/loss of electrons from the ionic solution, it is possible to draw detailed conclusions about its chemical status, ionic concentration, kinetic behavior, mechanism of reaction, and more.
Accurately characterizing ionic solutions via electrochemical analysis requires a series of electrodes working in tandem. At XRF Scientific, we have years of experience manufacturing anodes and electrodes for complex analytical applications. Our suite of platinum products includes a choice of electrodes suitable for various forms of electrochemical testing.
In this article, we will briefly explore the working principles of electrochemical analysis before outlining a few different platinum products suitable for electrode applications.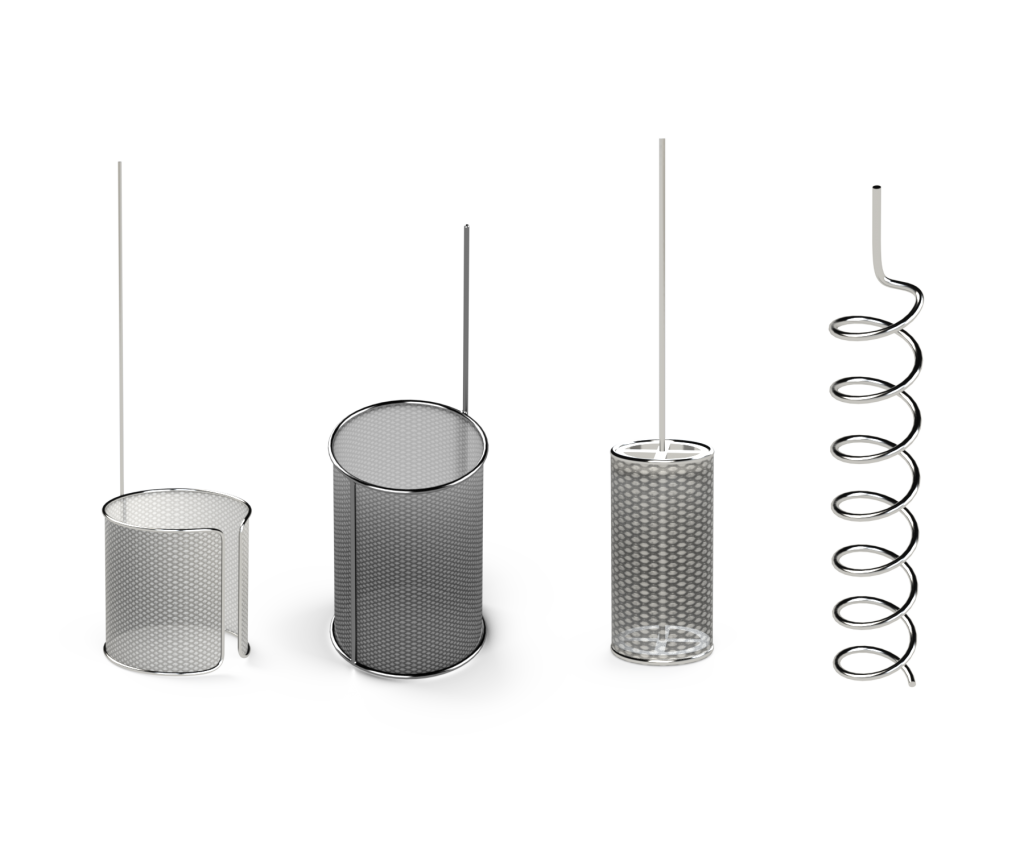 Electrochemical Analysis: Electrode Materials, Parameters & Techniques
Electrochemical Analysis: Electrode Materials, Parameters & Techniques
Given the sheer variety of electrochemical techniques available (cyclic voltammetry, square wave voltammetry, etc.), it should come as no surprise that a choice of substrate materials can be used in electrochemical cells. Platinum products, alongside other noble metals, are routinely used for electrode substrates due to their high chemical stability and ease-of-manufacture.
Three different electrodes are used in a typical electrochemical cell configuration, though this may vary depending on the experiment parameters – which further determines which technique to use for specific ionic solutions.
Of these three electrodes (working, reference, and auxiliary), the working component is the most important as it represents the interface where electrochemical reactions occur. This means the electrode must be immersed in the ionic solution under an applied current, which provides the basis for determining the reaction rate as a function of the number of ions produced/reduced per unit interface area over time.
Platinum products are among the preferred materials for working electrodes in electrochemical cells, though they have also demonstrated outstanding performance as reference and auxiliary components too.
Types of Platinum Electrodes from XRF Scientific
XRF Scientific designs and develops a choice of electrodes for electrochemical experiments based on our suite of tried-and-trusted platinum products. Our standard format electrodes are engineered to suit various electrochemical cell formats, including open and closed-front cylinders and wire helix type electrodes. We also provide custom Shoniger basket-types upon request.
We also offer a range of sample carriers based on high-purity platinum alloys. If you would like more information about product specifications, simply contact a member of the XRF Scientific team today.









